Camelidae IgG antibodies
Characteristics of the camelidae (camel, llama) antibody synthesis
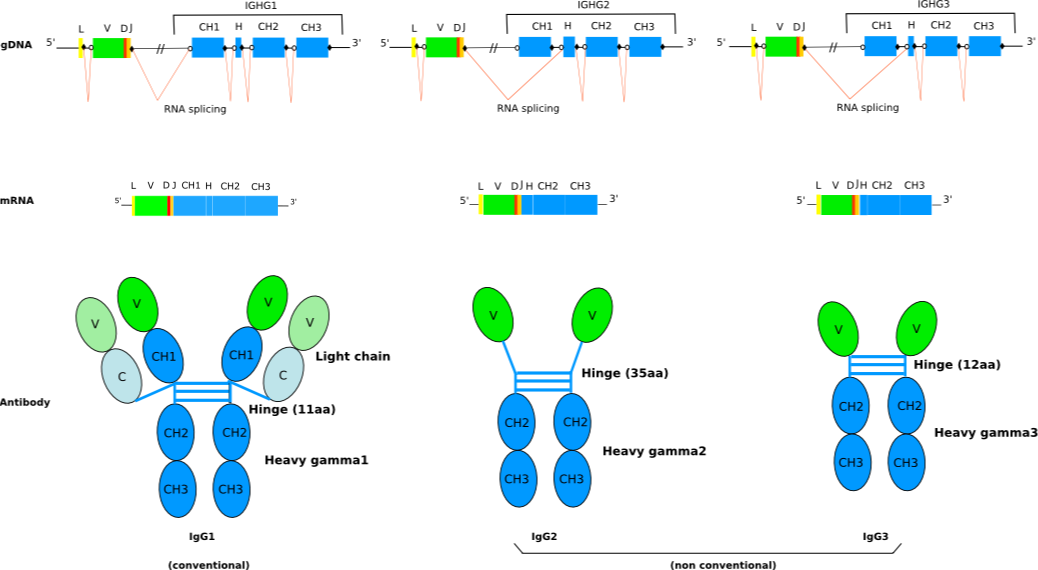
The Arabian camel (Camelus dromedarius) and the llama (Lama glama) have three IgG subclasses: the conventional IgG1 and the non-conventional IgG2 and IgG3.
IgG2 and IgG3 are characterized by the absence of light chain. This lack of light chain is itself a consequence of the absence of the CH1 domain in the gamma 2 and gamma 3 chains, due to a splicing defect of the CH1 exon (non canonical DONOR-SPLICE: nat instead of ngt) (Gene table: IGHC camel).
This situation is similar to that found in human in Heavy Chain Diseases (HCDs)
The Camelidae IGHV genes (Gene table: IGHV camel, IGHV llama, Protein display: IGHV camel, IGHV llama) belong to the IGHV3 subgroup and comprise two sets, the first set (IGHV3S1 to IGHV3S41) observed in the gamma 1 chains of the conventional IgG1 and the second set (IGHV3S42 to IGHV3S74) observed in the gamma 2 and gamma 3 chains of the nonconventional IgG2 and IgG3.
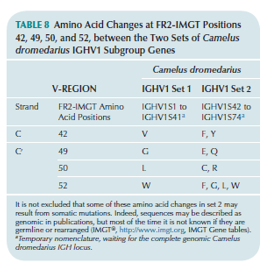
The Camelidae IGHV genes of second set genes (IGHV3S42 to IGHV3S74) show four amino acid changes at positions 42 (F, Y), 49 (E, Q), 50 (C, R) and 52 (F, G, L, W) according to the IMGT unique numbering, which make the C' strand more hydrophilic (IMGT Colliers de Perles). This co-evolution of the variable region (more hydrophilic) and of the constant region (absence of CH1 due to a mutation in the splicing site) is particularly remarkable.
Interestingly, the CDR3-IMGT of the VH domains (V-D-J-REGION) that involve V genes of the second set is longer than the average length of 13 amino acids, usually observed for conventional antibodies. As the antibody specificity is confered by a single domain, these VH domains also referred as VHH domains (for VH of only-heavy-chain IG) are frequently used in antibody engineering.
See also:
- Gene table: Arabian camel (Camelus dromedarius) IGHV (IMGT Repertoire)
Dromedary IgG2 and IgG3 only-heavy-chain antibodies
Excerpt of:Lefranc M-P. IMGT® immunoglobulin repertoire analysis and antibody humanization.
In: Alt, F.W, Honjo, T, Radbruch A. and Reth, M. (Eds.), Molecular Biology of B cells, Second edition, Academic Press, Elsevier Ltd, London, UK, Chapter 26, 2014, PP. 481-514.
dx.doi.org, ISBN : 978-0-12-397933-9. LIGM: 438
page 507-508 (source to be quoted)
‘Two IgG antibody formats are expressed in the dromedary or Arabian camel (Camelus dromedarius) and in Camelidae in general: the conventional IG (with two identical heavy gamma chains associated to two identical light chains) and the ‘only-heavy-chain’ IG (no light chain, and only two identical heavy gamma chains lacking CH1) (96). The Camdro (for Camelus dromedarius in the 6-letter species abbreviation) IGHV1 genes belong to two sets based on four amino acid changes which are characteristic of each set (97). The first set of IGHV1 genes is expressed in conventional tetrameric IgG1 that constitute 25% of circulating antibodies. The second set is expressed in the only-heavy-chain antibodies, IgG2 and IgG3 that constitute 75% of the circulating antibodies (96). The four amino acid changes are located in the FR2-IMGT at positions 42, 49, 50 and 52, the first position 42 is in the C strand and the three others (49, 50 and 52) in the C′ strand (Figure 1, Table 8). They belong to the [GFCC′C″] sheet at the hydrophobic VH-VL interface in conventional antibodies of Camelidae as well as of any vertebrate species whereas, in camelid only-heavy-chain antibodies (no light chains, and therefore no VL), these positions are exposed to the environment with, through evolution, a selection of hydrophilic amino acids.
The respective heavy gamma2 and gamma3 chains are both characterized by the absence of the CH1 domain owing to a splicing defect (98). It is the absence of CH1 which is responsible for the lack of association of the light chains. Only-heavy-chain antibodies is a feature of the Camelidae IG as they have also been found in the Bactrian camel (Camelus bactrianus) of Central Asia and in the llama (Lama glama) and alpaca (Vicugna pacos) of South America. The genetic event (splicing defect) responsible for the lack of CH1 occurred in their common ancestor before the radiation between the 'camelini' and 'lamini', dating approximately 11 million years (Ma) ago.
The V domains of Camelidae only-heavy-chain antibodies have characteristics for potential pharmaceutical applications (e.g., easy production and selection of single-domain format, extended CDR3 with novel specificities and binding to protein clefts). They are designated as VHH when they have to be distinguished from conventional VH (the sequence criteria is based on the four amino acids at positions 42, 49, 50 and 52). The term 'nanobody' initially used for describing a single-domain format antibody is not equivalent to VHH, as it has been used for V domains other than VHH and for constructs containing more than one V domain (VH and/or VHH) (e.g., caplacizumab, ozoralizumab) (IMGT® http://www.imgt.org, IMGT Repertoire > Locus and Genes > Gene tables; ibid., IMGT Biotechnology page > Characteristics of the camelidae (camel, llama) antibody synthesis; ibid. IMGT/mAb-DB > caplacizumab; ibid. IMGT/mAb-DB > ozoralizumab).’
(97) Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hamers, C., Bajyana Songa, E., Bendahman, N., and Hamers, R. (1993). Naturally occurring antibodies devoid of light chains. Nature 363, 446-448.
(98) Nguyen, V.K., Hamers, R., Wyns, L., and Muyldermans, S. (2000). Camel heavy-chain antibodies: diverse germline VHH and specific mechanisms enlarge the antigen-binding repertoire. EMBO J. 19, 921-930.
(99) Nguyen, V.K., Hamers, R., Wyns, L,. and Muyldermans, S. (1999). Loss of splice consensus signal is responsible for the removal of the entire CH1 domain of the functional camel IgG2a heavy chain antibodies. Mol Immunol. 36, 515-524.
Last updated: Friday, 06-Feb-2026 15:23:15 CET
Authors: Yan Wu and Marie-Paule Lefranc
Editor: Chantal Ginestoux
IMGT Home page |
IMGT Repertoire (IG and TR) |
IMGT Repertoire (MH) |
IMGT Repertoire (RPI) |
IMGT Index |
IMGT Scientific chart |
IMGT Education |
IMGT Latest news ![]()



© Copyright 1995-2026 IMGT®, the international ImMunoGeneTics information system® | Terms of use | About us | Contact us | Citing IMGT

